Blog
Dental Implant Complications: When We Know Better, We Do Better
September 30, 2025 Clinical Reena M Talwar, DDS PhD FRCD(C)
Reena M Talwar, DDS PhD FRCD(C)
Clinical Case Overview
Patient Presentation:
A 47-year-old healthy female patient presented to the clinic with complaints of discomfort and occasional swelling around a dental implant in the region of tooth #42 (lower right lateral incisor). The implant had been placed approximately one year prior to her complaint. The restoration of the implant was performed by an external provider. Initial osseointegration and function had been reported as successful. The implant was clinically, radiographically, and ISQ tested prior to sending her back to the referring dental clinic for restorative care. Over the past several months, post-implant restoration, the patient had noticed bleeding while brushing the implant, mild purulent discharge, and gingival pain and swelling.
Clinical & Radiographic Examination Sequence
Findings:
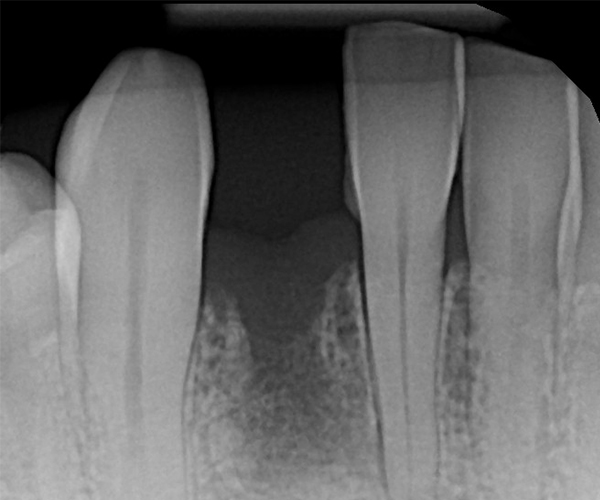
Clinical examination revealed inflamed peri-implant soft tissue, probing depths of 6–8 mm circumferentially, and mild suppuration. No mobility was noted, but the buccal tissue appeared slightly recessed with grey shadowing of the gingival marginal tissues. A periapical radiograph was obtained and showed significant vertical bone loss around the implant extending apically, with crater-like defects on all aspects.
Prosthetic Evaluation:
The implant prosthetic, which was deemed to be fabricated using off-label prosthetic components, was also poorly designed with a blunt over-hanging abutment at the implant platform and evidence of a cemented prosthetic. The contact areas with the adjacent teeth were long and non-cleansable. Given the extent of the defect and related prosthetic issues, it was deemed that the implant had a poor long-term prognosis and was determined to be failing due to advanced peri-implantitis.
Treatment Decision:
After a thorough discussion with the patient outlining the diagnosis, treatment options, and associated risks, the decision was made to remove the implant, graft the site, and allow for delayed implant replacement. The patient consented to the procedure and was scheduled for surgical intervention.
Surgical Procedure
Initial Surgery:
Under local anesthesia and intravenous conscious sedation, a full-thickness mucoperiosteal flap was raised to fully expose the implant and surrounding bone defect. The implant crown was removed, and the implant itself was atraumatically removed using a thin fissure with sterile saline irrigation and a reverse-torque technique to minimize trauma to the alveolar bone and prevent further bone loss. Granulation tissue was debrided using mechanical curettage and copious sterile saline irrigation was performed. The circumferential defect was measured at approximately 6 mm on the mesial and 8 mm on the mesial aspect of the implant with iatrogenic removal of the buccal cortical plate prior to implant removal.
Guided Bone Regeneration:
Guided bone regeneration (GBR) was performed using an allograft particulate bone substitute (a mineralized/demineralized blend). The graft material was carefully packed into the defect to reestablish the alveolar ridge contour. A resorbable collagen membrane was then placed over the graft to protect the site and enhance regeneration. The flap was advanced, with periosteal release, and secured with tension-free closure using PTFE sutures.
Post-Operative Care:
Post-operatively, the patient was prescribed a 7-day course of amoxicillin and instructed to rinse twice daily with 0.12% chlorhexidine. Post-operative instructions included gentle brushing of the surgical site using a Curaprox post-surgical brush, maintaining a soft diet, and returning for regular follow-up. Healing was uneventful with no complications. Sutures were removed at 10 days, and follow-ups at 1.5 and 3 months showed good soft tissue maturation and stable ridge form.
Implant Replacement:
At 4 months post-grafting, a CBCT scan demonstrated sufficient bone volume and density to support a new implant at site #42. A 3.5 x 10 mm narrow-diameter Nobel Biocare TiUltra CC implant was placed at 25 Ncm torque, with ISQ testing measuring 68 and a 3.6 x 3 mm healing abutment inserted at 15 Ncm torque. After 4 months of osseointegration, the implant was restored with a screw-retained zirconia crown, using on-brand Nobel Biocare prosthetic components.
1-Year Follow-Up
Outcome:
At the 1-year follow-up, the implant was functional with healthy surrounding tissues and no signs of inflammation. The implant–bone crestal bone level was maintained due to improved prosthetic design: good emergence profile, short well-positioned contact areas, and screw-retained prosthetic with proper contours.
Key Takeaway
This case underscores the importance of utilizing branded implant and prosthetic components, following evidence-based surgical and prosthetic principles. It also highlights the importance of diagnosis and treatment planning for failing dental implants treated with either an implant salvage technique versus implant removal, grafting, and replacement treatment protocol.
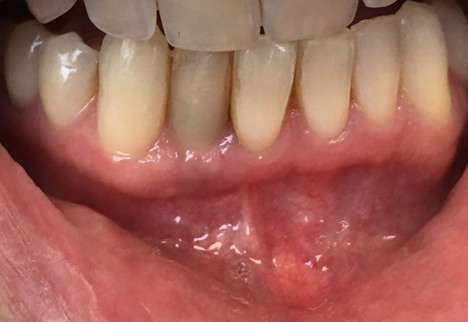
figure 1.
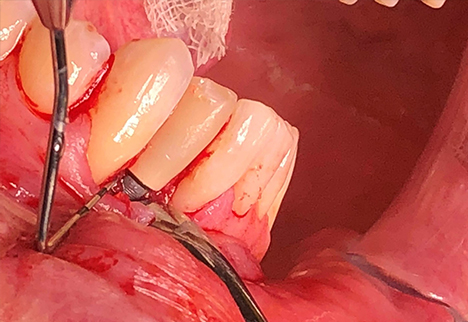
figure 2.
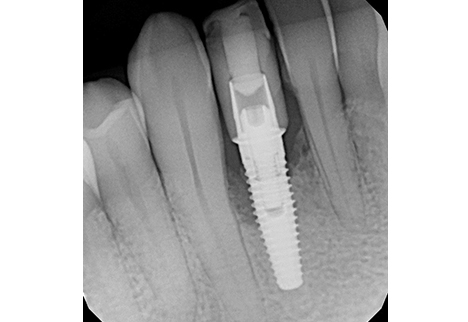
figure 3.
Archive
Business

January 27, 2026

October 24, 2023

August 2, 2023

October 1, 2022
Clinical

September 30, 2025

December 11, 2024

August 15, 2023

July 12, 2023

April 7, 2023

January 20, 2023
Giving Back

March 24, 2024

October 2, 2023

February 10, 2023
Health And Wellness

January 27, 2026

March 24, 2024
Lifestyle

March 10, 2023

February 7, 2023

October 12, 2021
Join Our Community!
Sign up to get Women in Dentistry news and updates delivered to your inbox.
By submitting this form, you are granting Women in Dentistry Inc., permission to email you.
You may unsubscribe via the link found at the bottom of every email.




